KITE-PWS is an ongoing Phase II trial of RGH-706 for people with PWS ages 17 and up. The study is sponsored by Gedeon Richter, a Hungarian-based company with 120 years of drug development experience. They have produced more than 200 drugs in over 40 countries, and have 12,000 employees worldwide.
The KITE-PWS study aims to evaluate an experimental drug, RGH-706’s effects on hyperphagia in adults with PWS. RGH-706 blocks a hormone in the hypothalamus in the central nervous system as its signaling promotes food-seeking behavior. People with PWS have an imbalance between the checks and balances of melanin-concentrating hormone and orexigenic signaling, so it is believed that by blocking this receptor, we might have an opportunity to decrease hyperphagia. The KITE-PWS trial will help us understand if RGH-706 safely and effectively blocks this receptor and decreases hyperphagia.
What are the objectives of the study?
The primary endpoint for the KITE-PWS study is decreased hyperphagia. The validated hyperphagia questionnaire for clinical trials, which is the gold standard questionnaire used in previous clinical trials with PWS, will be used to measure changes in hyperphagia.
This study will evaluate both the safety and tolerability of RGH-706 and will gather information about the pharmacokinetics of the drug. The study will also explore the effect of the drug on body weight composition; and on a set of different metabolic biomarkers. Questionnaires will also be used to learn if GH-706 can positively affect caregiver burden.
Who can participate in the study?
- Must have a genetic diagnosis of PWS
- At least 17 years old
- Body weight between 88 and 450 lbs
- Stable body weight for the past months (weight change ≤5% in the previous 3 months)
- Consistent and reliable caregiver for the past 3 months or longer
- No uncontrolled diabetes or diabetes that requires insulin
- Medical history and other criteria will be reviewed to determine eligibility*
How is the study designed?
The KITE-PWS study is divided into two parts: Part A and Part B. Part A requires 60 people with PWS and will be 23 weeks in duration. The treatment period will last 6 weeks and requires 6 on-site visits as well as a set of telehealth visits. Part B is a 21 weeks commitment with a 15-week treatment period and will require 100 participants. An interim analysis will take place between the two parts to look at the data and tailor the study as needed to best capture the effects of the drug. In both parts A and B, study participants will be randomly assigned drug or placebo and will take an oral capsule once daily.
At this time, there is not an open-label extension but it is the company's hope that they will be able to offer an extension at a later date.


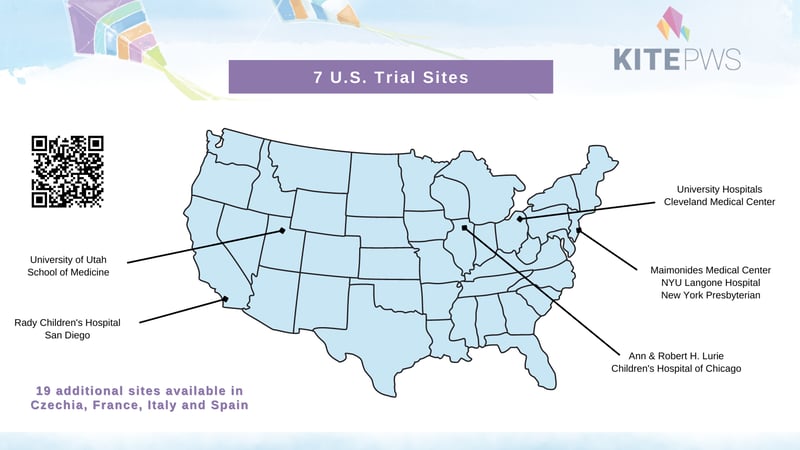
Where are the trial sites?
KITE-PWS is an international study with sites across the US, Czechia, France, Italy, and Spain. A complete list of sites is available on clinicaltrials.gov. Participants will be reimbursed for trial-related travel and accommodations.
What are the next steps if I want to participate in the trial?
Interested caregivers should contact Gedeon Richter at medinfo@richter.hu or contact the trial site most convenient to them to request a screening.







