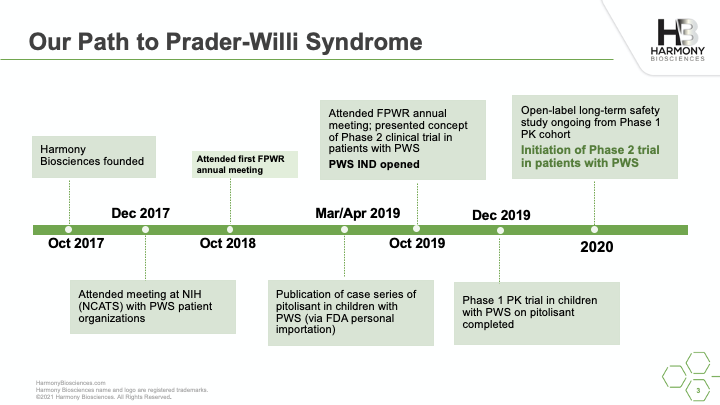
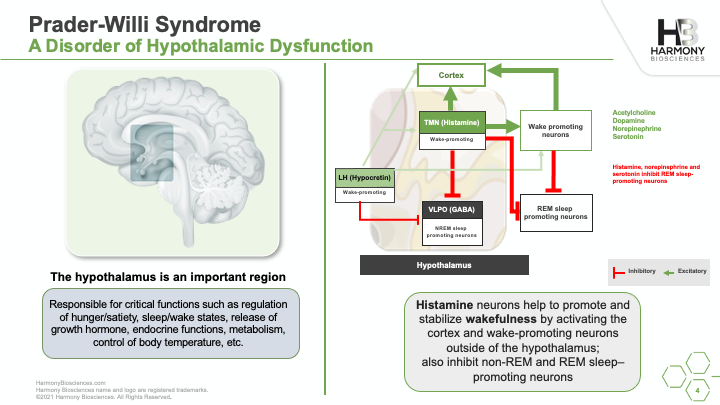
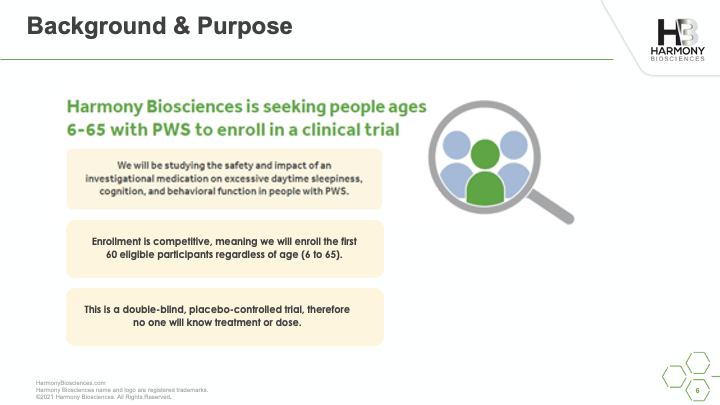
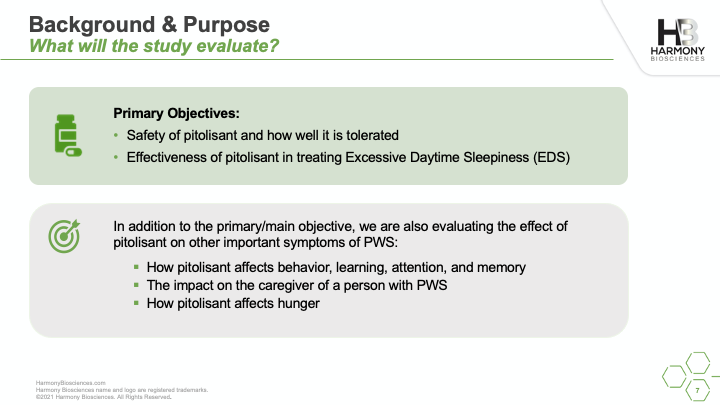
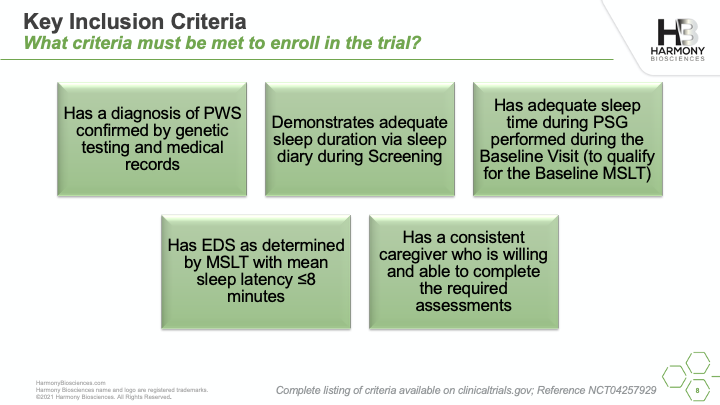
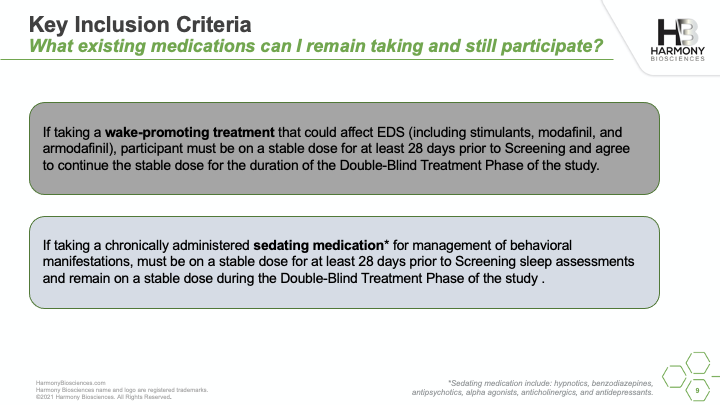
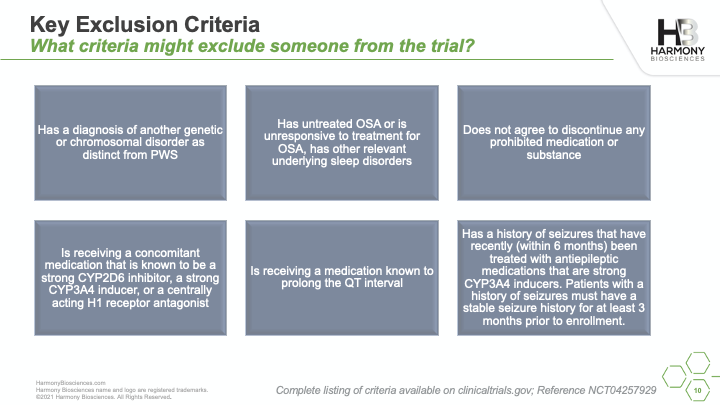
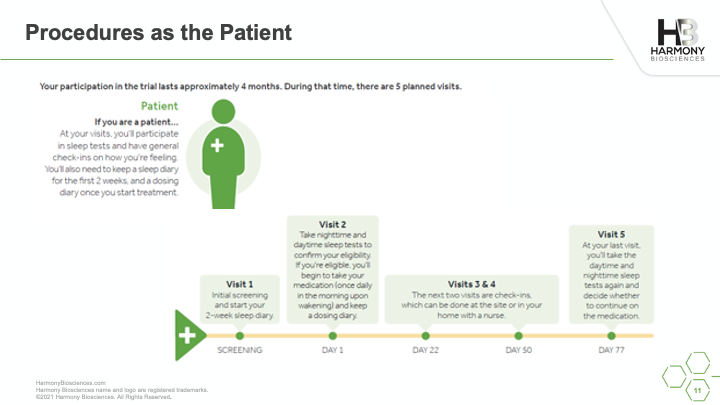
A phase 2 study of Pitolisant is enrolling patients ages 6 to 65 years old to evaluate the safety and impact of an investigational medicine, Pitolisant, for excessive daytime sleepiness, cognition, and behavioral function in people with PWS. A live webinar was conducted in July 2021 sharing details of the study, what you can expect if you choose to participate, and eligibility criteria for participation.
The presentation is 24 minutes in length, followed by 10 minutes of Q&A. You can watch the complete presentation by clicking on the embedded video. In case you don't have time to watch the full video, we've included slides from the presentation below.