Topics: Research
Results have now been published on a study looking at eye tracking and hyperphagia in younger children with PWS (ages 3-11 years old). The study found that children with PWS who had higher hyperphagia questionnaire scores and more advanced nutritiona...
Two recent papers address an area that has received very limited attention to date, aging in PWS. Both papers point to differences in aging in young adults with PWS compared to typical individuals and suggest that this is an area that is in need of f...
Topics: Research
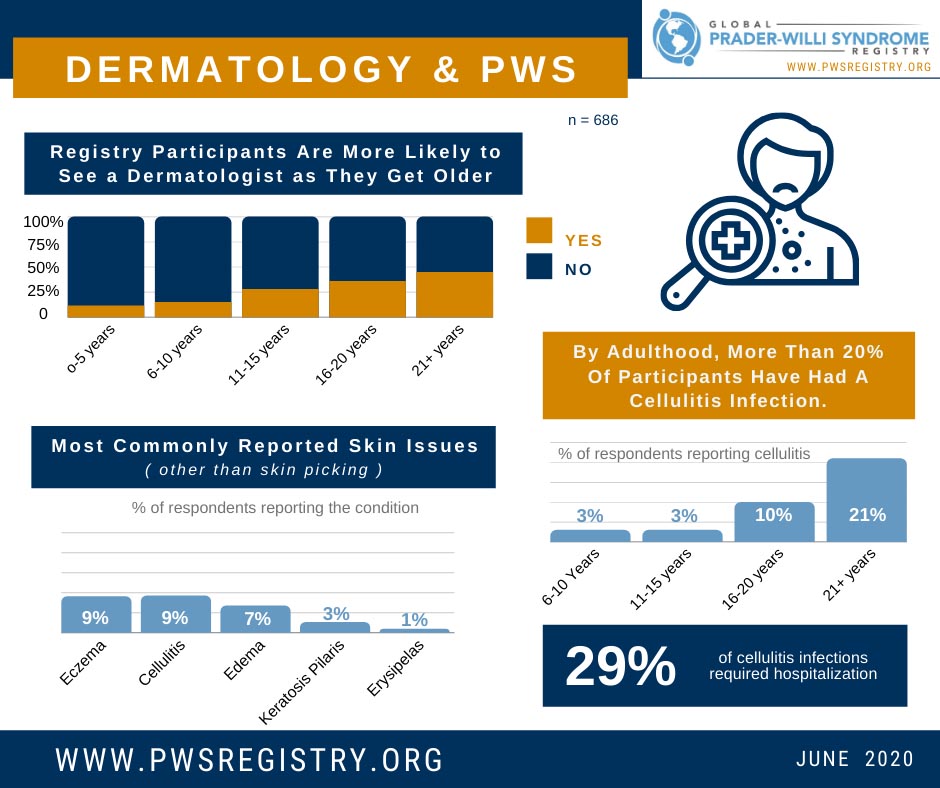
When it comes to dermatological or skin issues in PWS, the first things that may come to mind are sensitivity to the sun due to fair skin, and skin picking. However, there are a variety of additional skin-related concerns for individuals with PWS. Da...
Topics: Research
Soleno Therapeutics has announced top-line results from the company’s phase 3 trial, DESTINY PWS (C601), evaluating once-daily diazoxide choline controlled release (DCCR) tablets for patients with Prader-Willi syndrome (PWS). The results of the study...
Topics: Research
A special contribution by guest blogger Alice Shapley Our beautiful and amazing 7‑year‑old daughter Anna has Prader‑Willi syndrome (PWS). There are so many things for which I am incredibly, deeply proud of Anna, but today I want to talk about the rol...
Topics: Stories of Hope
The Foundation for Prader-Willi Research announces our first round of Research Awards in 2020 totaling $912,251. FPWR is dedicated to supporting research that advances the understanding and treatment of Prader-Willi syndrome (PWS) and to that end, ha...
Topics: Research
COVID-19 has made all of our homes feel a bit smaller these days... Living together 24/7 has surely whittled away at our patience and increased everyone's anxiety. In this webinar, Patrice Carroll, Director of PWS services at Latham Centers, gives ad...
Topics: Research
With COVID-19 keeping many of us indoors and isolated from our friends and family, we are all seeking new ways to stay connected and spice up our days! A few innovative community members have shared fun activities they have found that allow them to c...
Topics: News
A new pilot project is seeking funding to map the genomes of 50 people with PWS and integrate that information with the Global PWS Registry data. This first-ever PWS Genome Project seeks to help researchers better understand differences in PWS sympto...
Topics: News