Topics: News
With COVID-19 keeping many of us indoors and isolated from our friends and family, we are all seeking new ways to stay connected and spice up our days! A few innovative community members have shared fun activities they have found that allow them to c...
A new pilot project is seeking funding to map the genomes of 50 people with PWS and integrate that information with the Global PWS Registry data. This first-ever PWS Genome Project seeks to help researchers better understand differences in PWS sympto...
Topics: News
COVID-19 has made all of our homes feel a bit smaller these days... Living together 24/7 has surely whittled away at our patience and increased everyone's anxiety. In this webinar, Patrice Carroll, Director of PWS services at Latham Centers, gives ad...
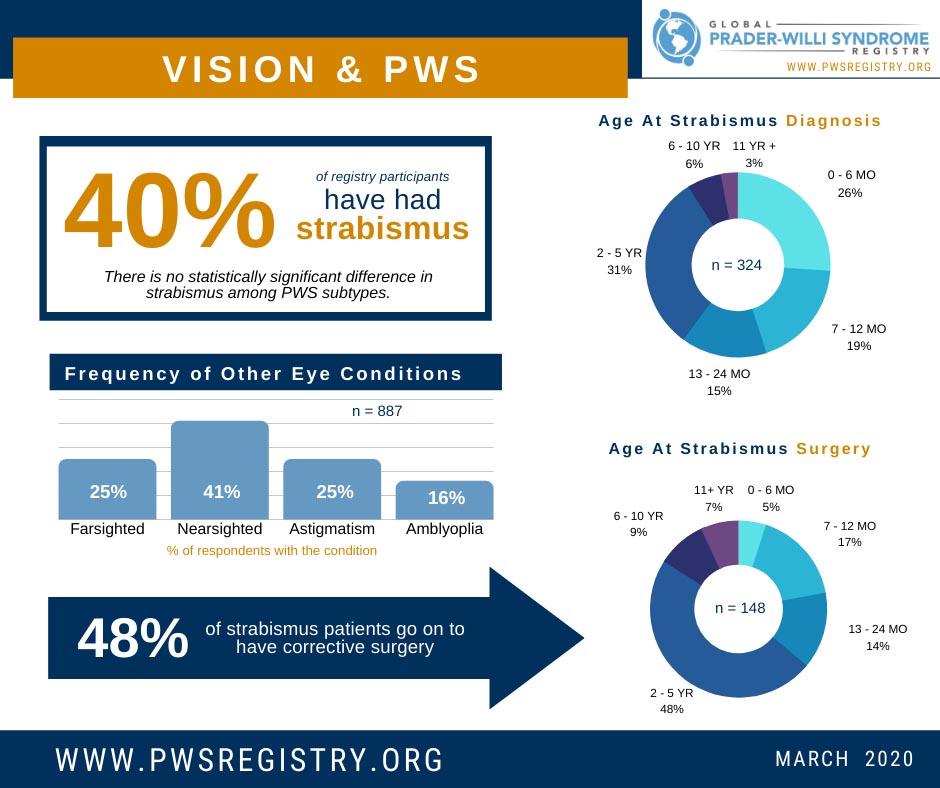
Although many symptoms of PWS are difficult to treat, impaired vision is a place where corrective lenses and/or surgery can make huge improvements. Data from the Global PWS Registry shows that the most common vision issues in PWS are nearsightedness,...
Topics: Research
Most of us are not trained educators, yet with COVID-19 closing schools around the country and many of us are facing distance learning for the remainder of the school year. In this webinar, Elizabeth Roof, Senior Research Specialist at Vanderbilt Res...
An Update to the Prader-Willi community from Harmony Biosciences: As you may know, the team at Harmony has been working toward initiating a Phase 2 clinical trial to assess the safety, efficacy and appropriate dosing of pitolisant in treating some of...
A special contribution by guest blogger Felicia DiMuccio Growing up, I was never quite sure what I wanted to be, but I always knew I wanted to be a mom. Finding out I was pregnant with Siena was a dream come true, and I couldn't wait for her to enter...
Topics: Stories of Hope
Today we received disappointing news from Millendo in regards to their Phase 2 study of Livoletide in PWS (Zephyr). Unfortunately, results from the trial do not show the significant improvements in hunger that we had all hoped to see and Millendo has...
In our latest podcast, Patrice Carroll, Director of PWS Services at Latham Centers in Cape Cod, shares how to how to help our loved ones with PWS through this unusual time of social distancing and isolation due to COVID-19. To listen, just click abov...
Topics: Research