Topics: Research
Data from the PATH for PWS study, an ongoing natural history study of individuals with PWS sponsored by the Foundation for Prader-Willi Research, supports the benefits observed in the open-label study (C602) of DCCR. Analysis of trial data has shown ...
Levo Therapeutic’s New Drug Application (NDA) for carbetocin as a treatment for PWS has been scheduled for a public meeting of the Psychopharmacologic Drugs Advisory Committee to be held on November 4th, 2021. The Food and Drug Administration (FDA) c...
Topics: Research
Dr. Rachel Wevrick and her team have just published a new paper looking at the function of the protein encoded by the MAGEL2 gene, one of the genes in the PWS region of chromosome 15. This is the first-ever study to look at the first part of MAGEL2. ...
Topics: Research
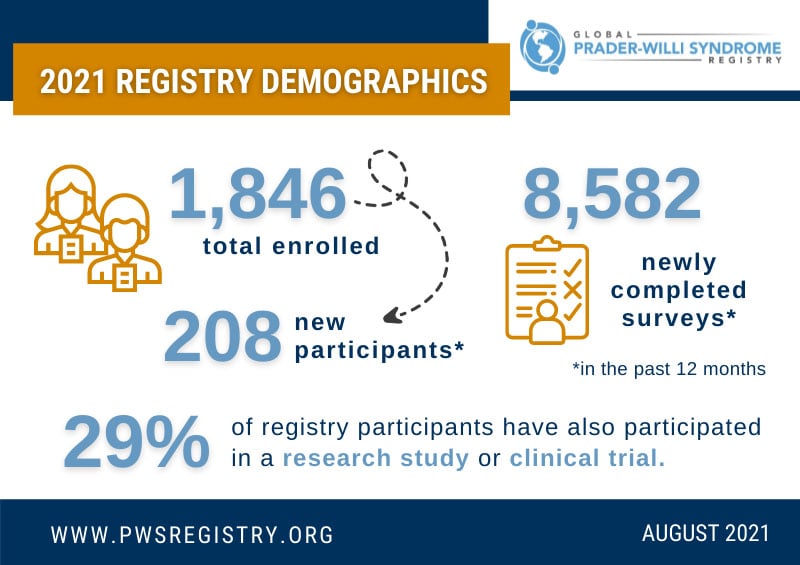
The Global PWS Registry continues to grow and strengthen as a valuable resource and research tool for the PWS community. The Registry currently has 1,846 participants. Over the past 12 months (July 2020-July 2021), the Registry has grown by over 12% ...
Topics: Research
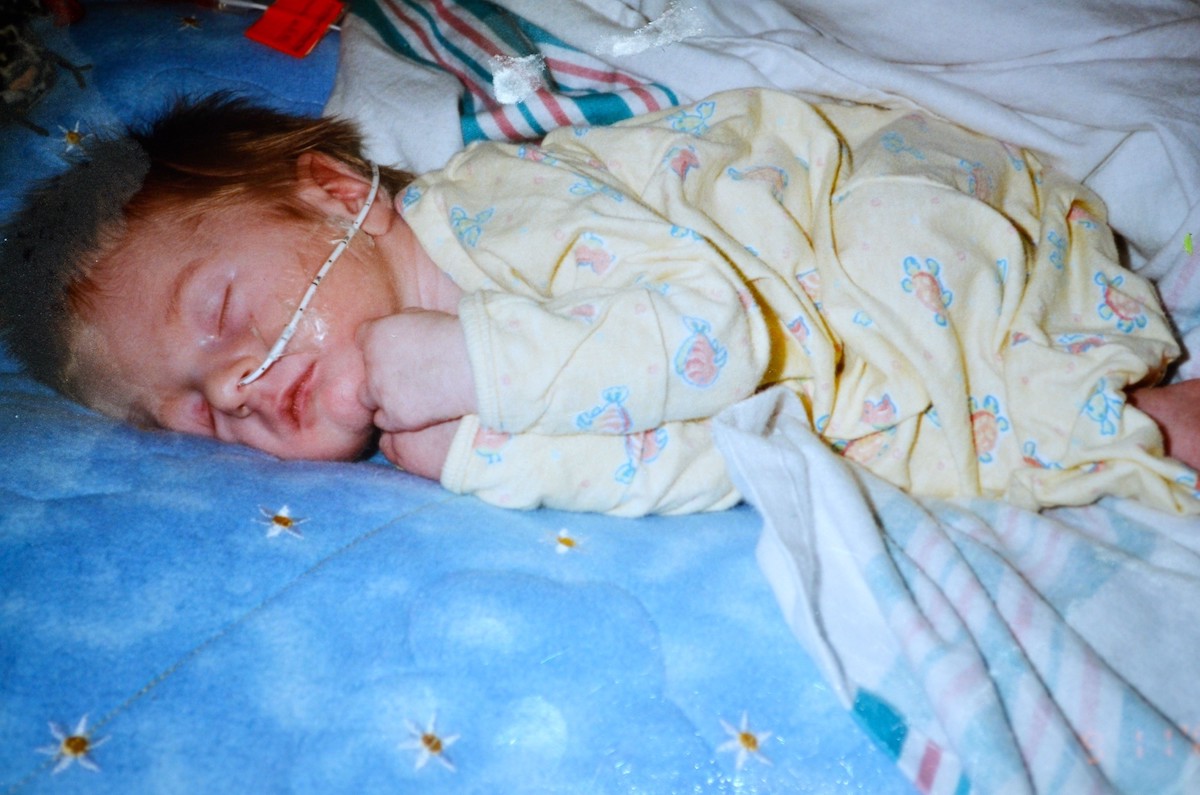
A special contribution by guest blogger Diane Schantin When Austin was born, our whole family was up at the hospital celebrating. I vividly remember the nurses coming in to check his vitals, watching their faces and hearing, “Oh my God.” His blood su...
Topics: Stories of Hope
Thanks to the participation of so many in our community, our team was able to share the largest study ever on eye problems in PWS, using data from the Global PWS Registry. The paper “Incidence of strabismus, strabismus surgeries, and other vision con...
Topics: Research
A special contribution by guest blogger Lisa Matesevac Our youngest child, Evan, was born in 2006. Little did I know what big changes were coming in our lives. I knew we would be outnumbered with three children. I knew we would have sleepless nights....
Topics: Stories of Hope
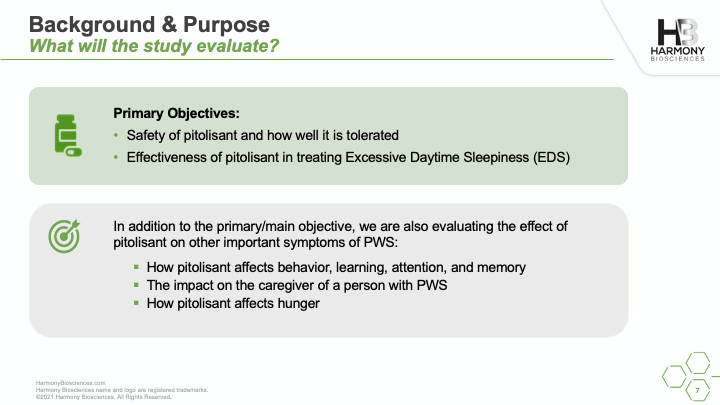
A phase 2 study of Pitolisant is enrolling patients ages 6 to 65 years old to evaluate the safety and impact of an investigational medicine, Pitolisant, for excessive daytime sleepiness, cognition, and behavioral function in people with PWS. A live w...
Topics: Research
On June 17, 2021, FPWR and PWSA | USA engaged the FDA in a Patient-Led Listening Session to share our community’s experiences related to Prader-Willi syndrome (PWS). The purpose of this meeting was to promote dialogue between the FDA and members of t...
Topics: News