Topics: Research
In this 1 hour and 12‑minute video, Dr. Katy Chambers, former high school principal, teacher, and mom to Daniel who lives with PWS, shares her journey maneuvering the special educational system from both sides of the table. The session includes Q&...
To ensure that new treatments for PWS are advanced as quickly as possible, FPWR collaborates with academic researchers, pharma companies, and other stakeholders to speed the drug development timeline and get treatments into the hands of patients fast...
Topics: Research
Physical activity and exercise are an important part of care for individuals with PWS. However, this can be difficult due to poor muscle tone and orthopedic issues. Many individuals require bracing, casting, and or surgery for spinal issues. Here, we...
Topics: Research
In this 1 hour and 2‑minute video, Dr. Theresa Strong, FWPR’s director of research programs, shares highlights from the 2021 PWS Research Symposium. The session includes Q&A from participants in the 2021 FPWR Virtual Conference.
Topics: Research
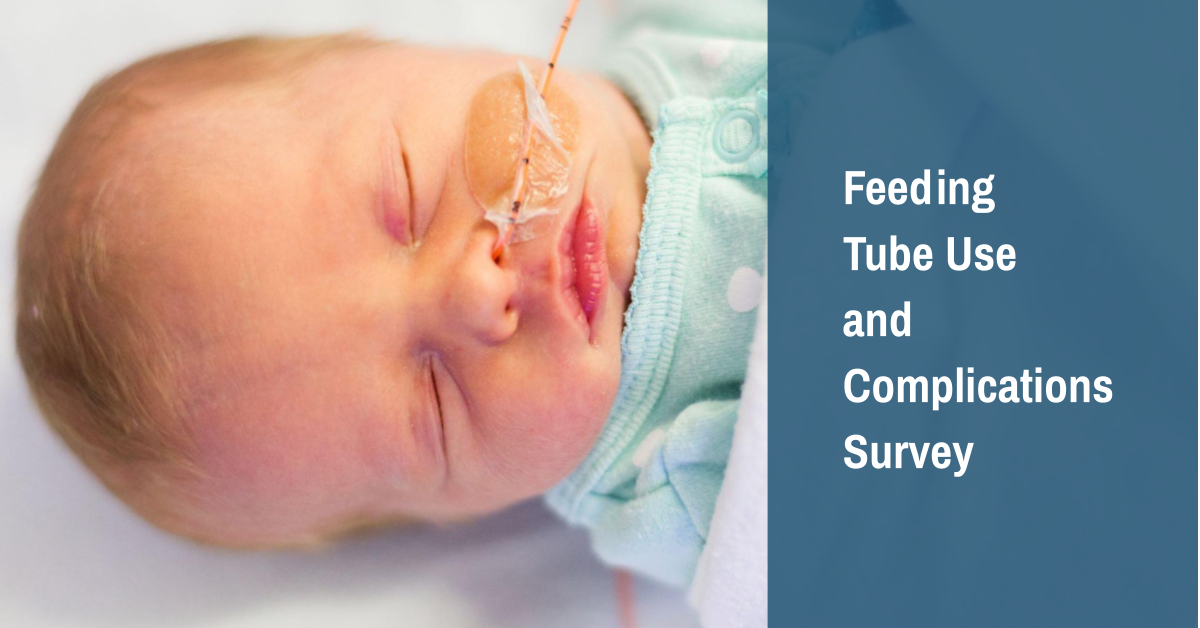
Researchers from the PWS CLIC (Clinical Investigation Collaborative) need your help to learn more about the use of feeding tubes in infants with PWS.
Topics: Research
With the support of the John C. Storr and Suzanne M. Storr Foundations, FPWR is excited to announce the launch of three new research programs designed to translate findings from the lab to human clinical trials, accelerating the development of new tr...
Topics: Research
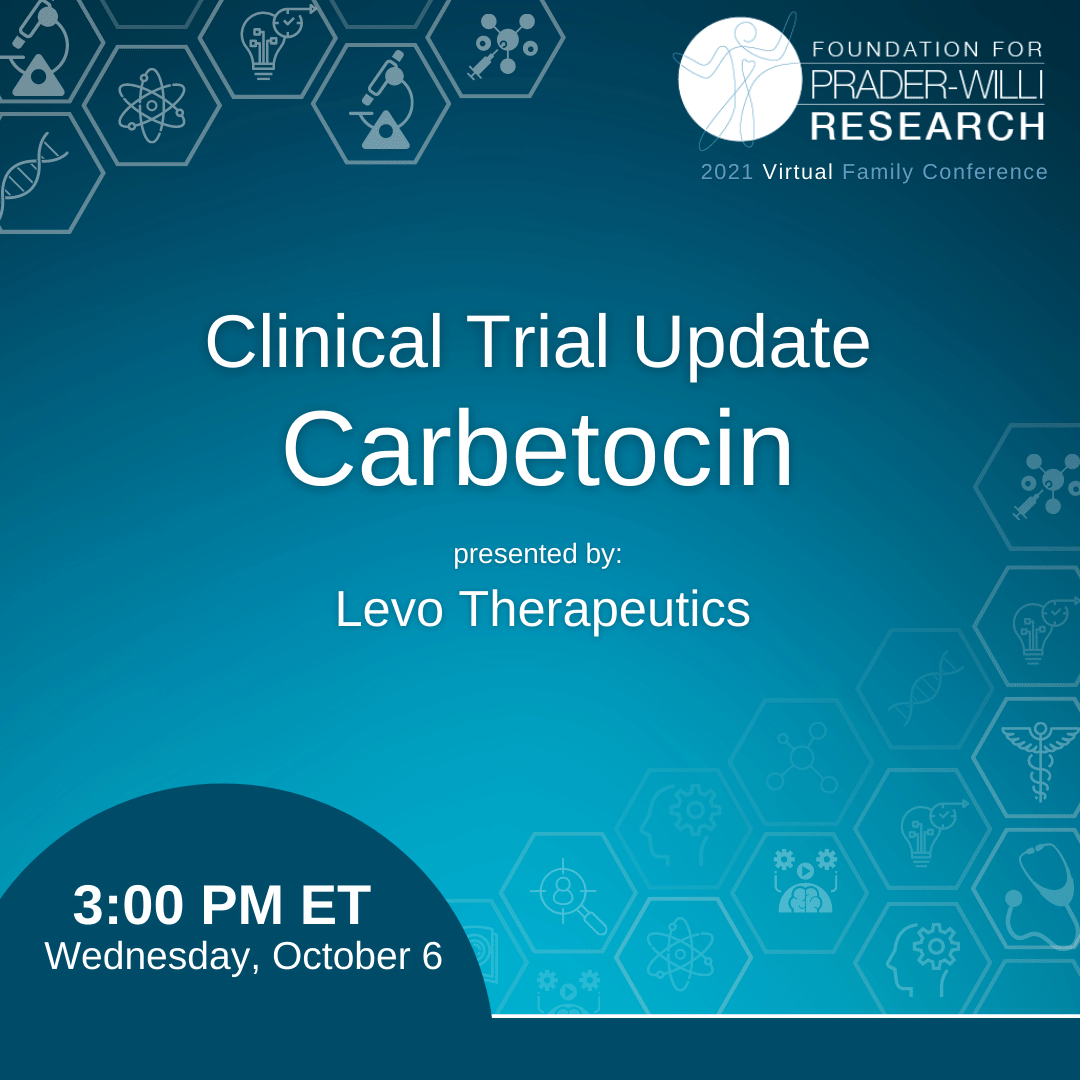
In this 35‑minute video, Sara Cotter, founder and CEO of Levo Therapeutics, joins other members of the Levo team in describing the company’s mission of developing impactful therapies for the PWS community. The session includes Q&A from participan...
Topics: Research
Harmony Biosciences recently announced changes to their Phase 2 Clinical Trial of Pitolisant that reduce the requirements of participating in the study.
Topics: Research
A special contribution by guest blogger Jeannine Kowal, FPWR Board President Share your story via our Stories of Hope Questionnaire. Caitlin's birth was uneventful. Caitlin's birth was induced at thirty‑nine and a half weeks because of growth concern...
Topics: Stories of Hope