ENROLLING NOW!

Does your loved one struggle with disruptive behavior and temper outbursts? They could be eligible to participate!
This trial will evaluate the effectiveness of a wearable medical device in treating temper outbursts and disruptive behaviors.
Seeking participants AGES 10-40 with a history of TEMPER TANTRUMS over the past 6 months.
ONLY 2 IN-PERSON VISITS REQUIRED!

VNS4PWS is a phase 3 clinical trial evaluating the effectiveness of a Vagus Nerve Stimulation (VNS) device for improving disruptive behaviors and temper outbursts for people with PWS. If your loved one is between the ages of 10 and 40, you may be eligible to participate!
.png?width=800&height=1000&name=VNS%20temper%20tantrums%20(2).png)
Eligible participants will wear a tVNS device for 4 hours each day and the caregiver will respond daily to a short 1-3 question survey to provide data on the frequency and severity of the day's outbursts. Only two in-person visits are required for the 12-month study. FPWR has partnered with Angel Flight NE to provide free travel (flights and amtrack) to and from clinical visits.
About VNS
About VNS
VNS uses brief electrical impulses to stimulate areas of the brain that are important for controlling emotions and behavior.
An implantable VNS device has been approved by the FDA to treat epilepsy, chronic recurrent depression, migraines, and cluster headaches. Both implanted and wearable VNS devices have been studied in a small number of individuals with PWS with a significant reduction in both the number and severity of temper outbursts.
The potential benefits of VNS in people with PWS were first demonstrated using a surgically implanted VNS device in three adults with PWS. While no effects on hyperphagia or weight were observed, improvements in temper outbursts were seen in two of three adult participants. The participant who showed no improvement did not have a prior history of temper outbursts. The two participants whose behavior improved asked to continue with the VNS after the study finished, and in an informal follow-up eight years later both individuals reported continuing benefits and no unexpected side effects.
A second clinical trial using transcutaneous vagus nerve stimulation (tVNS) has also shown efficacy. The external device was worn for four hours each day with an electrode in the left ear to stimulate the auricular branch of the vagus nerve. Temper outbursts improved significantly in 4 out of 5 participants after six to nine months of tVNS. (Manning et al., PLOS One. 2019). By the end of the 9-month treatment period, significant reductions in the number of temper outbursts were observed to the extent that participants that responded to the tVNS treatment had no outbursts or, at the most, the very occasional outburst. No significant side effects were observed.
The VNS Device
.png)
About the VNS Device
The device used in our VNS4PWS clinical trial is:
- Small and portable
- Attaches to your ear like an earbud
- Worn for 4 hours/day while going about your daily activities.
The tVNS device is a small device that can be easily worn as part of your daily routine. During the study the device will need to be worn for a total of 4 hours each day. This time can be done at once, or split up throughout the day.
Eligibility
Who Can Participate in the Trial?
- People with PWS ages 10-40 and their caregivers
- Have a history of disruptive behaviors over the past 6 months
- Medications have been stable for the past 90 days
- Not currently participating in another clinical trial (medication or behavioral)
Participants who complete the trial will have the option of enrolling in an extension study so they may continue to use the device!
You can learn more about the VNS4PWS trial in this short webinar:
Locations
Locations
Find A Site Near You
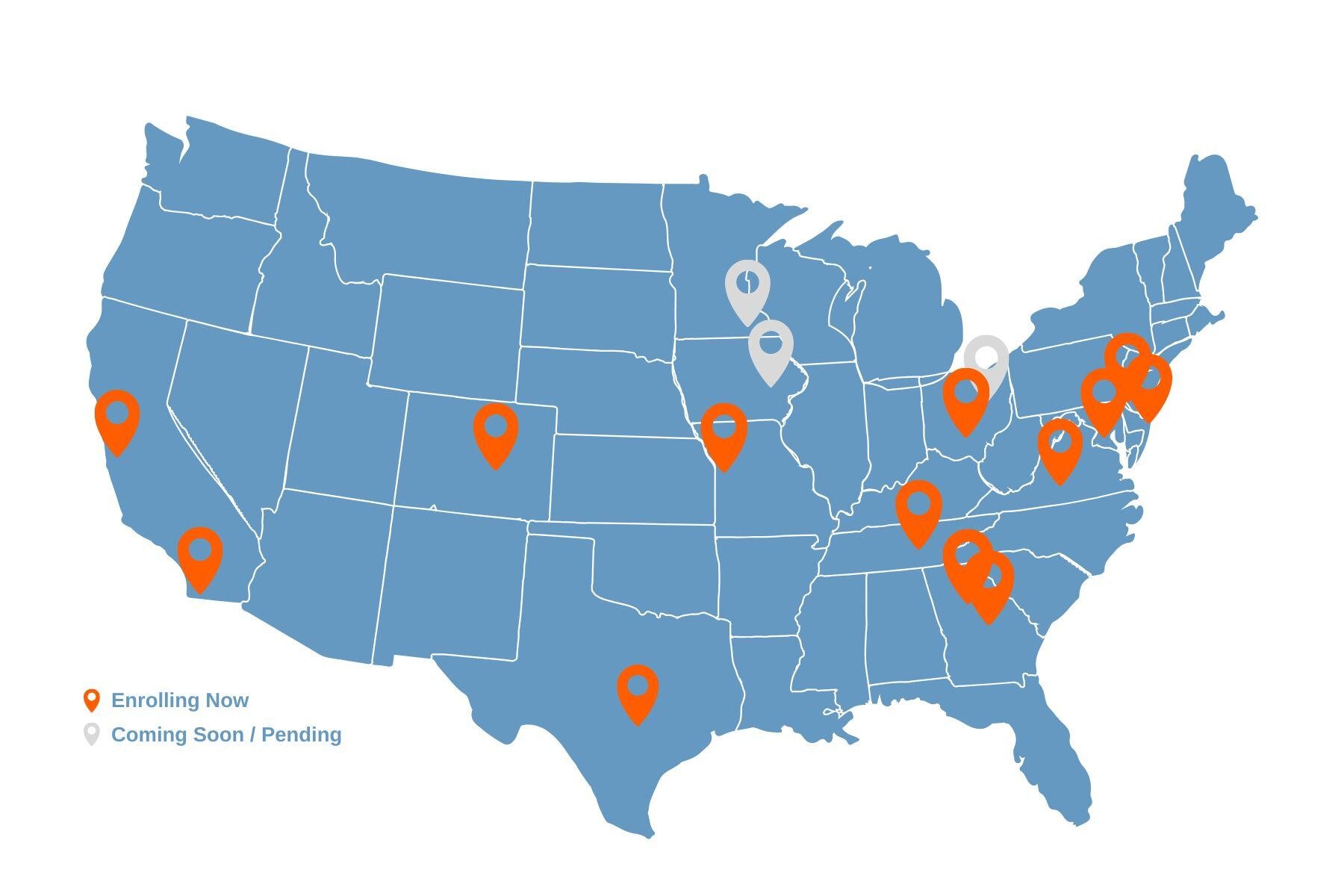
ENROLLING NOW:
Atlanta, GA
Baltimore, MD (ages 10-19)
Bronx, NY
Brooklyn, NY
Charlottesville, VA
Columbus, OH
Denver, CO
Kansas City, MO (ages 10-19)
Mineola, NY
Nashville, TN
Palo Alto, CA
San Antonio, TX
San Diego, CA
COMING SOON:
Cleveland, OH
Iowa City, IA
Minneapolis, MN
Contact Us
Contact Us
Questions? Ready to schedule your screening appointment?
Email us at VNS@fpwr.org or complete this form and our trial coordinator will contact you.
